A group of researchers from the American MIT university has improvements on lithium-air batteries have been developed, that the capacity of the batteries increase. The researchers for this purpose, carbon nanofibers in which as electrode function.
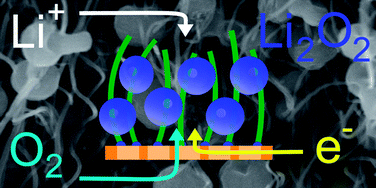
A lithium-air battery uses oxygen from the atmosphere as oxidizer of lithium, in contrast to a lithium-ion battery, where the cathode is a solid substance. In the formation of lithium oxide, energy is released as electrical energy could be utilized. A lithium-air battery is lighter, because no solid, but a porous cathode, with oxygen continuously through air can be brought in. The energy density per unit weight of such a battery is greater than a lithium-ion battery.
The amount of lithiumperoxide which are located on the cathode can be binding is the limiting factor for the energy that the battery can deliver. However, the internal surface of the cathode to increase, it can be the bindingsoppervlak, and thus the capacity, can be expanded. The MIT researchers have this is achieved by cathodes of carbon nanodraden with a diameter of 30nm to produce. The electrodes contain more than ninety percent empty space, write the researchers in their paper.
The energy density per unit weight of their batteries would 2500Wh/kg a record and more than four times larger than that of a traditional lithium-ion battery. The accutechniek works for now only in the lab; a commercial product has yet to be developed. It provides the technique, whereby the nanoparticles are arranged neatly, a possibility of the reactions in the electrodes in detail with an electron microscope to study and gain more insight into the battery’s to obtain them to further improve.